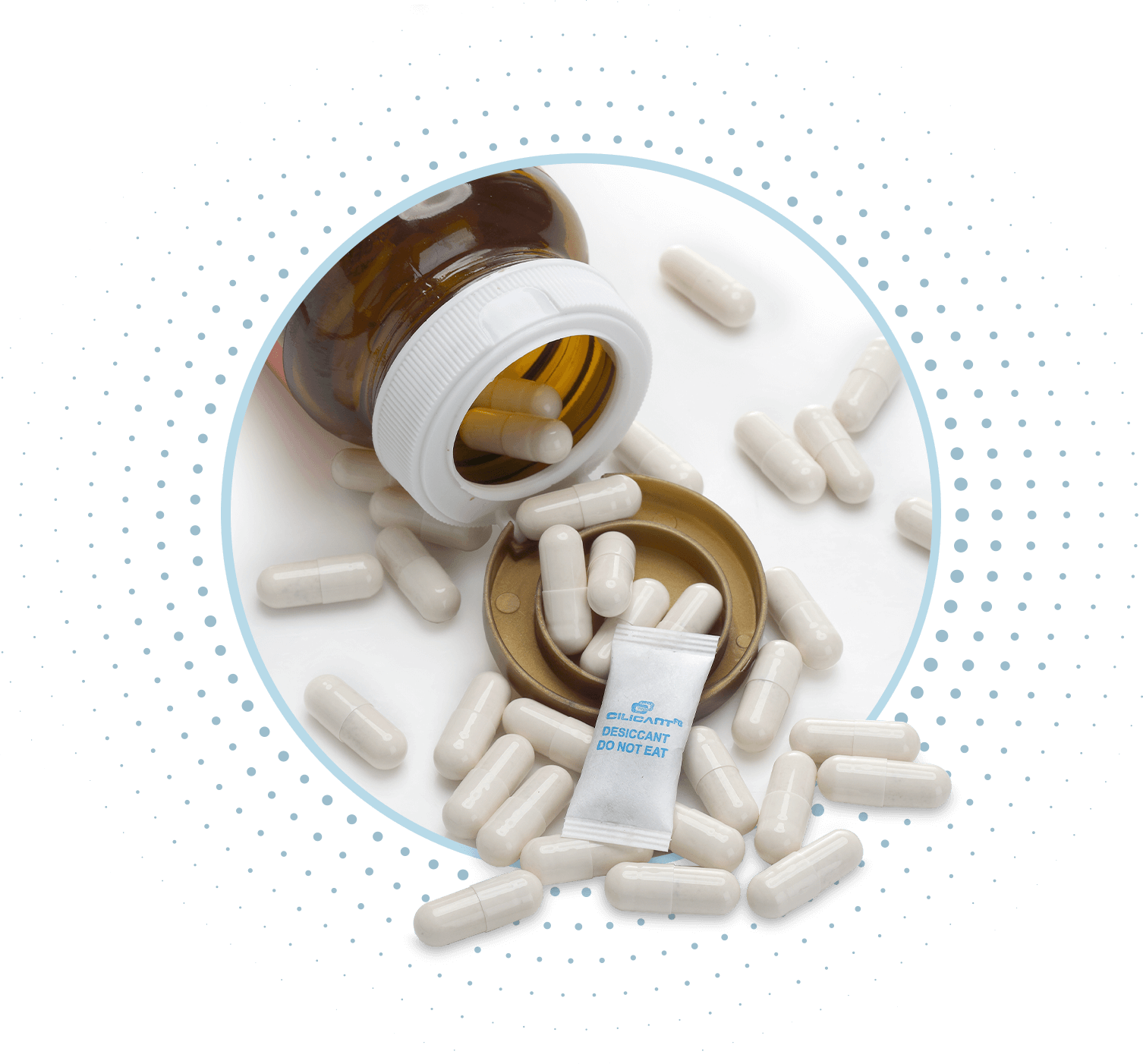
Overview
CILICANT FG Desiccant Pouches can be safely used in direct contact with a wide range of pharmaceuticals and nutraceuticals. All CILICANT FG Desiccant Pouches are manufactured in ISO 15378 cGMP facility, adhering to stringent global standards. As such, they are compliant with 21 CFR. They are tested in compliance with current USP 41 <670> guidelines. CILICANT FG Desiccant Pouches also hold both a Type III DMF (Drug Master File) and a Health Canada Master File (MF).
Features
Active Sorbent Fills
CILICANT FG pouches consist of a variety of active sorbents including:

Silica Gel (moisture control)

Molecular Sieve (moisture control)

Activated Carbon (odour control)

Custom Blend (moisture and/or odour control)
CILICANT FG desiccant pouches are available in the following configurations:
- Pre-cut (0.5 g – 10 g) *
- Strips (0.5 g – 3 g) *
- Bags